Learn more about the advantages of ultraviolet absorption of proteins
There are two main methods for detection of proteins and protein crystals. One is to image or acquire spectra of the intrinsic fluorescence of the proteins. The second is to image or acquire the absorption spectra of the proteins. Of the two, the second is better especially for the identification and analysis of protein crystals.

Ultraviolet absorbance image of a protein crystal in solution
1. Speed
Proteins usually absorb strongly at 280 nm. This means that images and spectra can be acquired quickly. On the other hand, the intrinsic fluorescence of proteins is weak in comparison, meaning that exposure times must be long.
2. Damaging Ultraviolet (UV) light
Weak fluorescence means that protein samples must be exposed to damaging UV light for longer periods of time. Absorbance imaging and absorption spectroscopy is completed rapidly so UV exposure is short and limited.
3. Tryptophan required for fluorescence
The residue tryptophan has the highest fluorescence quantum yield of the amino acids that fluoresce. If it is not present in a high enough concentration, there is no detectable fluorescence. With absorption, tryptophan is just one amino acid of many that absorb UV light. Additionally, the peptide bonds between the amino acids also absorb in the deep UV!
4. Fluorescence can be quenched
Impurities, other amino acids, and even the protein's structure can potentially quench the fluorescence of the already weakly emitting trytophan residues.
5. Contaminants easily distinguished
Salt crystals and other contaminants are easily and rapidly distinguished from protein crystals by UV absorbance imaging and absorption spectroscopy.
6. Determination of protein concentration
UV absorption can be used to determine the concentration of the protein in a crystal.
7. Analysis of DNA and RNA
UV absorption can be used to analyze DNA and RNA samples and crystals whereas fluorescence cannot since neither DNA nor RNA fluoresce.
8. Determining the contamination of DNA and RNA samples
The contamination of DNA or RNA crystals by protein can be rapidly and safely measured in a non-destructive fashion using absorption methods which is not possible using protein fluorescence.
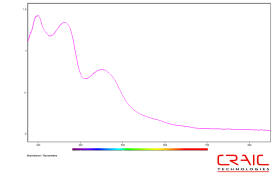
Absorption microspectra™ of a protein crystal

